 Waukeshahealthinsurance.com-
Waukeshahealthinsurance.com-
–
All her life, college student Olivia Cooke has had only a small amount of central vision. It was as if she was looking at the world through a straw, and in the dimly lit places she could make out only the figures of people, but their faces.
But after receiving an experimental gene-editing treatment for one of her eyes, she can now see things she's never seen before.
Cook was born with an inherited retinal disorder that causes blindness, a rare eye disorder historically known as Liber Congenital Amaurosis, or LCA. A few years ago, she decided to participate in a clinical trial using the gene-editing tool CRISPR to correct her hereditary blindness.
“My life has changed a lot in terms of the hope that there will be more science and discovery in the future,” said Cook, 22, who is currently studying marketing and product development at Missouri State University in Springfield. She received the experimental gene editing treatment through surgery on her left eye.
“Now after surgery and recovery, I can see dimly in my left eye,” Cook said.
Treatment using CRISPR In a phase 1/2 clinical trial in which Cook participated, it was found to be safe and effective in improving vision among younger patients with hereditary blindness.
Among a total of 14 volunteers, including Cook, the gene-editing device was found to be associated with a “significant improvement” in vision for most patients after about three months, and was not directly linked to any serious side effects, he said. The trial results, published Monday in the New England Journal of Medicine, say the treatment remains experimental and the results need to be replicated in a larger group of people.
After months of treatment, Cook sat on the porch with friends, Christmas lights wrapped around the railing. It was late, she remembered, but she could see her friends' faces glistening under the twinkling Christmas lights. She was shocked.
“I could not see their faces with my right eye. I could only see a picture of them. I could see everything in their faces with my left eye — so, a noticeable difference, especially in the dim light,” Cook said of that night.
“One of the biggest 'aha moments' I had was talking to my mom one day after the surgery — it was about six to nine months after the surgery that I noticed most of the improvement,” Cook said.
“I saw a candle shining behind me that I had never seen before,” she said. “I had never raised anything with the environment before.”

Before the treatment, Cook said she could sometimes hide the visual challenges she had. Her limited vision was often an internal struggle.
“Until you spend a lot of time with me, you don't know how terrible my eyesight is,” Cook said. “If we saw her on the street, if I introduced myself to you, you would never know her.
But now she no longer hides.
This study is the first to use CRISPR from the perspective of living humans.
“The results of this study provide proof of concept that CRISPR-Cas9 gene editing can be used to safely and effectively treat inherited retinal diseases,” said the study's first author, Dr. Eric Pierce, director of the Ophthalmic Genomics Institute at Mass Eye and Ear. and Harvard Medical School.
The trial was funded by the biotechnology company Editas Medicine and was conducted by researchers at the Mass Eye and Ear of the Mass General Brigham Health Care System and other US-based institutions, including the Perelman School of Medicine at the University of Pennsylvania. University of Michigan, University of Miami, and Oregon Health & Science University.
“We are really hopeful that CRISPR-Cas9 gene editing technologies will now be applied to other forms of hereditary blindness and other genetic diseases in general,” Pearce said. “We hope this will help usher in an era of therapeutic use of CRISPR-Cas9 technologies.”
In the year The trial, which began in 2019, enrolled 12 adults aged 17 to 63 and two children aged 9 and 14 with hereditary retinal degeneration. CEP290 gene. That gene provides instructions for making a protein involved in many types of cells, including light receptor cells in the eye. Mutations in CEP290 are the most common cause of early-onset retinal degeneration, leading to vision loss in children.
Currently, there is no US Food and Drug Administration-approved treatment for CEP290-associated hereditary retinal degeneration. These patients cannot read lines of letters or numbers on the vision chart that most people receive from an eye doctor, and the visual impairment may worsen over time.
For the trial, 14 participants were surgically injected under the retina of one of their eyes with a drug called Edit-101, which contains CRISPR gene-editing components. Since the trial was primarily conducted to evaluate safety and efficacy, only one eye was studied in each patient.
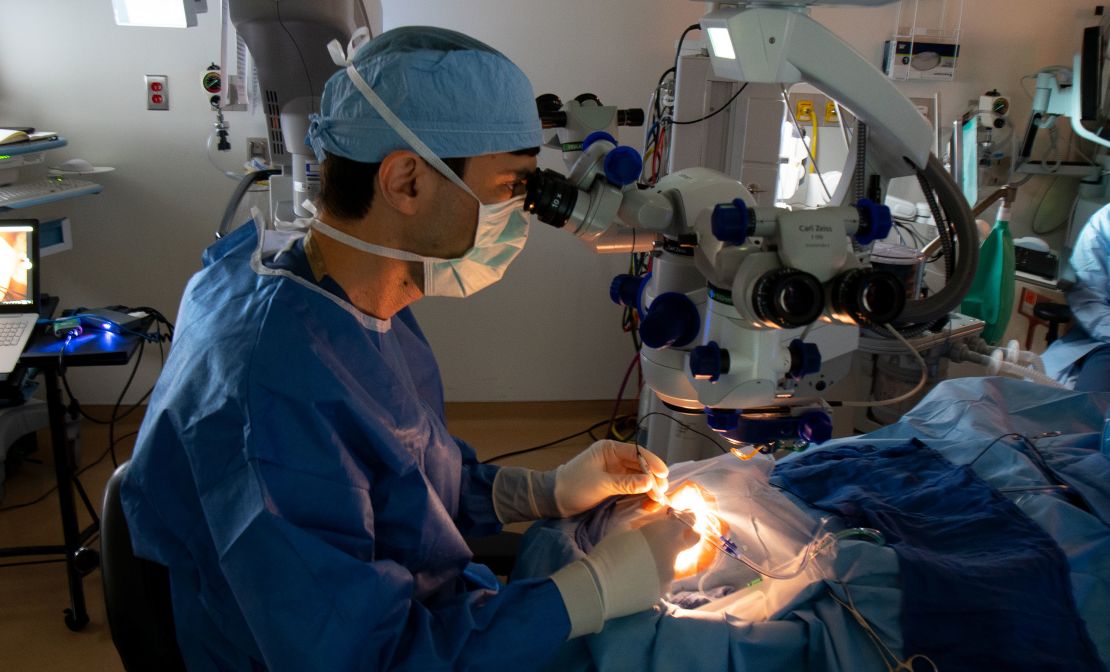
“The subjects received an injection of a gene-editing drug called EDIT-101 under the retina,” Pearce said. “That drug covers the CRISPR-Cas9 gene-editing machinery, and once it starts working in the patients' retinal cells, it cuts out the mutation in CEP290 from the genome of their retinal cells, allowing the CEP290 gene to function. It's back.”
The first patients in the study were treated in 2020 For the first time in the history of medicine, CRISPR-based medicine was used for gene editing directly to the living human body.
Of the adult volunteers, two were given the low dose, five the middle dose, and five the high dose. Both children in the study were given a medium dose. The outpatient procedure took about an hour and a half.
Then the patients were followed up every three months for one year and less follow up continued for two years. During these visits, they performed a series of visual tests, among other assessments.
The researchers found that 11 patients in the study experienced some improvement in their eyesight following CRISPR therapy, and that these improvements occurred approximately three months after the procedure and during follow-up visits.
Also, no serious side effects occurred in response to the treatment at any dose, according to the researchers, and the adverse events that occurred were mild or moderate. There was also no indication that CRISPR gene-editing caused ripple-effect damage to the patients' genomes.
“The primary goal of this first-in-human study was to test the safety of using CRISPR-Cas9 gene editing in vivo. When we started the trials, the people treated were the first patients to receive CRISPR-Cas9 gene editing therapies in vivo,” said Pierce. “There were no serious adverse events related to treatment or surgery required to deliver the treatment and no dose-limiting toxicities.”
After the operation, one patient experienced some bleeding in the eye, which impaired their vision, but that was later resolved, according to the researchers.
“Once that hemorrhage cleared, the subject's vision returned to normal,” Pierce said.
Another patient experienced visual impairment six months after surgery associated with small nodules under the retina. These types of hyperreflexive hills have been seen in studies of other subgene therapies, the researchers said, and their cause is unclear.
“It's supposed to be swelling,” Pierce said of the bumps.
According to the study, the patient was treated with a course of steroid medication and continued to recover.
“As the hills cleared, so did their vision,” Pearce said. “I think this drug is as safe as possible in terms of design.”
Full vision was not restored among patients. Most in the experiment could not read any line of the eye chart before the study, and only four of them had some improvement in this ability. But after receiving treatment, some patients reported being able to see their cell phones light up, distinguish different foods on their dinner plates, recognize the rotating Apple icon on a computer screen, or even notice a bright sunset.
“I started seeing what was described as an explosion of color,” said Michael Kalberer, 46. He received CRISPR treatment in his right eye And he first noticed improvements in his vision after about two to six months. He started his research in 2020.
“Seeing the strobe lights on the dance floor at my cousin's wedding was a great moment,” said Calberer, who says that if he hadn't received the treatment, he would have been a shadow on the dance floor. And flashing lights, and could not distinguish colors.
Kalber described CRISPR therapy as “groundbreaking” but cautioned that it is not a cure-all.
“It's not a cure,” said Kalberer, who still can't see normal text or photos on the screen. “My disease is still here. I will not disappear. I am not healed. … But it definitely slowed down the growth rate.
Pearce said he believes this approach to using CRISPR as a treatment for hereditary blindness can be re-examined in a larger and more diverse group of patients. All Phase 1/2 trial participants were non-Hispanic and white.
In 2022 Editas Medis has announced that it has stopped Further studies of CRISPR gene editing as a therapeutic approach in CEP290-related hereditary blindness have continued to follow the treated patients to this day instead of conducting further trials.
Recent results from the Phase 1/2 trial support moving forward with a Phase 3 trial and ultimately filing the therapy for FDA approval, Pierce said.
“We are working with Editas to identify an additional commercial partner for Phase 3 studies. We certainly hope this publication will be of interest to the biotech and pharma communities,” Pearce said.
More…
